Direct C–H heteroarylation by an acenaphthyl-based α-diimine palladium complex: improvement of the reaction efficiency for bi(hetero)aryls under aerobic conditions†
Abstract
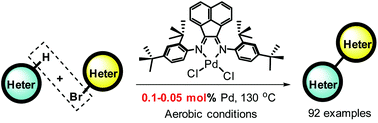
A bulky ancenaphthyl skeleton-based α-diimine palladium complex with ortho-tert-butyl on N-aryl moieties was designed, synthesized and characterized. The developed palladium complex was applied for direct C–H arylation under aerobic reaction conditions. A range of heteroaryls, such as thiozoles, thiophenes, furans, imidazopyridines, indolizines, isoxazoles, imidazoles, triazoles, pyrazoles, indoles, pyrroles and pyrazolidinones, were used, while various coupling partners of heteroaryl bromides with wide functional groups were compatible. Upon using 0.1–0.05 mol% of a precatalyst, more than 90 examples of cross-coupling products were afforded in good to excellent yields, demonstrating that this phosphine-free catalytic system scaffold enables a general access to biheteroaryls.


 Please wait while we load your content...
Please wait while we load your content...