Fluoride-mediated alkoxylation and alkylthio-functionalization of halogenated perylenediimides†
Abstract
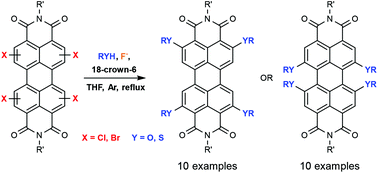
The reaction of ortho- and bay-substituted chloro- or bromoperylenediimides with alcohols and thiols in the presence of fluoride ions affords the corresponding alkoxy- or alkylthioPDIs in good to excellent yields under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...