Sodium nitrite-promoted aerobic oxidative coupling of aryl methyl ketones with ammonium under metal-free conditions: a facile access to polysubstitution imidazoles†
Abstract
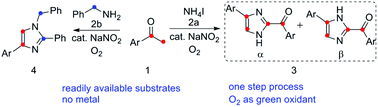
A practical, economic and efficient sodium nitrite-promoted aerobic oxidative synthesis of polysubstitution imidazoles from aryl methyl ketones has been developed. (4 or 5)-Aryl-2-aroyl-imidazoles are generated in good yields under metal-free conditions and in a one step process, which show highly potent antiproliferative activity. Based on some control experiments, a possible mechanism was proposed. Moreover, other multisubstituted heterocyclic derivatives, such as 1,2,4-trisubstituted imidazoles, were prepared under the sodium nitrite-promoted aerobic oxidative conditions.


 Please wait while we load your content...
Please wait while we load your content...