Synthesis of a ceria-supported iron–ruthenium oxide catalyst and its structural transformation from subnanometer clusters to single atoms during the Fischer–Tropsch synthesis reaction†
Abstract
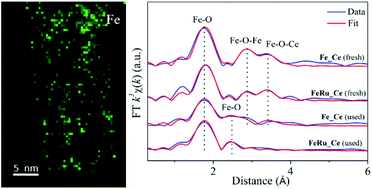
The formation of supported metal/metal oxide single-atom catalysts (SAC), as well as their structural evolution during catalytic reactions have attracted much research interest in the fields of both inorganic chemistry and catalysis recently. In this work, we report the synthesis of iron (ca. 10 at%) oxide catalysts with the doping of a small amount (0.5–0.6 at%) of ruthenium oxide, which have been deposited onto the surface of ceria nanorods by an optimized deposition–precipitation (DP) route. Multiple characterization studies including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM) and nitrogen adsorption/desorption confirmed the identical structural and textural properties of the ceria support after the DP step. Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) combined with electron energy loss spectroscopy (EELS) showed the formation of subnanometer iron species in the fresh samples. Furthermore, the X-ray absorption fine structure (XAFS) technique with the help of related data analysis verified the generation of noncrystalline iron oxide clusters predominantly composed of Fe3+ ions. Here, the addition of a secondary metal (ruthenium) greatly promoted the dispersion of Fe over the ceria nanorods. After the catalytic reaction of Fischer–Tropsch synthesis (FTS), the transformation from subnanometer iron oxide species to ionic Feδ+ single atoms has been revealed and confirmed by the corresponding profile fits on the extended X-ray absorption fine structure (EXAFS) spectra. In contrast to the normal coarsening process, the FTS conditions (up to 300 °C, 2 MPa, CO/H2 = 1/1) did drive the creation of such iron single atoms solely coordinated by oxygen ions.


 Please wait while we load your content...
Please wait while we load your content...