One-pot electrochemical preparation of copper species immobilized poly(o-aminophenol)/MWCNT composite with excellent electrocatalytic activity for use as an H2O2 sensor†
Abstract
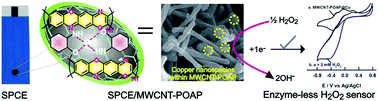
Redox activity of copper species immobilized poly(o-aminophenol)/multi-walled carbon nanotube (Cu@MWCNT-POAP) composite is reported for direct electrocatalysis towards detection of H2O2. The Cu@MWCNT-POAP composite was prepared by a single step in situ electrochemical method. The as-prepared Cu@MWCNT-POAP composites were characterized by field emission scanning electron microscopy, Fourier transform infrared spectroscopy, Raman spectroscopy, X-ray diffraction, and electrochemical impedance spectroscopy. Cyclic voltammetry (CV) studies highlighted the good redox behavior of the Cu@MWCNT-POAP composite resulting from direct electron transfer behavior of the copper redox couple (Cu2+/Cu+). The Cu@MWCNT-POAP composite displayed superior electrocatalytic activity and high performance towards detection of H2O2 because of its large surface area and homogenous immobilization of Cu species on the MWCNT-POAP composite. The analytical sensitivity and detection limit of the sensor were 831 μA mM−1 cm−2 and 30 nM, respectively. In addition, bio-catalytic activity of the sensor was evaluated using the Michaelis–Menten constant. The Cu@MWCNT-POAP composite showed excellent stability and reproducibility, and good recoveries for determination of H2O2 in milk, bleach cream, and contact lens solutions.


 Please wait while we load your content...
Please wait while we load your content...