The effect of pendant group structure on the thermoresponsive properties of N-substituted polyesters†
Abstract
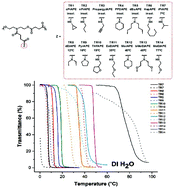
Synthetic polymers exhibiting reversible lower critical solution temperature (LCST), such as poly(N-isopropylacrylamide) (PNIPAM), are intriguing materials with various potential applications. We recently developed a new class of biodegradable thermoresponsive polyesters (TR-PEs) based on N-substituted diol monomers. TR-PEs exhibited reversible cloud point temperatures (Tcp) between 0–50 °C and were shown to be non-cytotoxic. The synthesis of N-substituted diols and TR-PEs is highly modular, allowing for a wide variety of possible homo- and copolyesters. In this work, we report the synthesis and characterization of 20 homopolyesters in order to better understand the structure–property relationship of TR-PEs. UV-vis spectroscopy showed that the Tcp of TR-PEs was highly dependent on pendant group structure, such as secondary or tertiary amides, cyclic and linear groups, and substitution of oxygen atoms. Structure-Tcp analysis provides a correlation between Tcp and the number of heteroatoms relative to the number of carbon atoms in the pendant group thereby providing a rationale for the design of thermoresponsive polyesters with desired Tcp values. To demonstrate the expanded tunability of the TR-PE system, copolyesters bearing covalently attached ibuprofen were synthesized and shown to exhibit LCST behavior dependent on comonomer hydrophilicity.


 Please wait while we load your content...
Please wait while we load your content...