Thermo and pH dual-responsive drug-linked pseudo-polypeptide micelles with a comb-shaped polymer as a micellar exterior†
Abstract
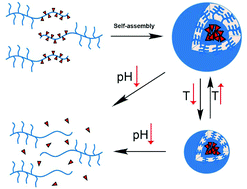
Herein, thermo and pH dual-responsive drug-linked pseudo-polypeptide micelles were prepared by a self-assembly strategy. Initially, a temperature-responsive comb-shaped poly(2-isopropyl-2-oxazoline)-b-linear poly(acrylic acid) block copolymer (CPiPrOx-b-PAA) was synthesized through a combination of living cationic ring-opening polymerization and living radical polymerization. Subsequently, conjugation of doxorubicin (DOX) with the linear PAA block through a pH-responsive acylhydrazone bond led to a block copolymer having an amphiphilic characteristic, and this block copolymer then self-assembled into CPiPrOx-b-PAA-DOX micelles. TEM and DLS measurements showed that the CPiPrOx-b-PAA-DOX micelles had a uniform spherical morphology with a solid size of about 50 nm and a hydrodynamic size of about 123 nm. Moreover, the micelle coating of comb-shaped poly(2-isopropyl-2-oxazoline) displayed a temperature-responsive behavior with a phase transition temperature of 45 °C. The CPiPrOx-b-PAA-DOX micelles showed a pH-responsive DOX release in vitro; this confirmed the acid-liable effect of the acylhydrazone bond that linked the linear PAA block with DOX. In in vitro cell test, a dose-dependent cytotoxicity and caveolae-mediated endocytosis of the CPiPrOx-b-PAA-DOX micelles were observed. Based on near-infrared fluorescence (NIRF) imaging, a significant tumor accumulation of the CPiPrOx-b-PAA-DOX micelles was observed when the dye-labeled CPiPrOx-b-PAA-DOX micelles were intravenously injected into H22 tumor-bearing mice.


 Please wait while we load your content...
Please wait while we load your content...