Mechanosynthesized copper(i) complex based initiating systems for redox polymerization: towards upgraded oxidizing and reducing agents†
Abstract
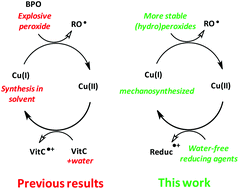
Significant improvements of the recently proposed Cu(I)/vitamin C + water/dibenzoyl peroxide (BPO) system for the redox free radical polymerization of methacrylates in air are presented here, the goal being to avoid the presence of water and to eliminate the unstable BPO compound. Additionally, the use of mechanosynthesized Cu(I) complexes bearing cheap triphenylphosphine and methylpyridine ligands, allowed us to reduce the economical and synthesis costs significantly. Water-free reducing agents such as 6-O-palmitoyl-L-ascorbic acid, organotin or hydrazine derivatives are evaluated. Cumene hydroperoxide and tert-butyl perbenzoate are proposed as a replacement for less stable BPO. Most of these novel combinations are more efficient in air than the usual amine/benzoylperoxide system (4-N,N-TMA/BPO) reference especially at the sample surface. Among them, Cu(I)/organotin/cumene hydroperoxide appears as an efficient and competitive alternative to the conventional amine/BPO couple.


 Please wait while we load your content...
Please wait while we load your content...