Synthesis of hyperbranched low molecular weight polyethylene oils by an iminopyridine nickel(ii) catalyst†
Abstract
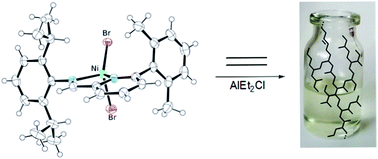
A 6-(2,6-dimethylphenyl)-2-(2,6-diisopropylphenyl)iminopyridine dibromo nickel(II) complex was synthesized, characterized by X-ray diffraction analysis and tested in ethylene polymerization using diethylaluminumchloride as the cocatalyst. Low molecular weight (Mn ∼ 103 g mol−1) polyethylene oils were obtained under a variety of reaction conditions. Detailed NMR analysis showed the formation of hyperbranched macromolecules (branching density >100 branches per 1000 carbons) with a high fraction of “branches on branch” and one unsaturation per chain, resulting in polymer features comparable to those of polymers produced by α-diimine Pd(II) catalysts. The DFT model of the catalytic species showed that the ortho-2,6-dimethylphenyl substituent of the pyridine group destabilizes the ethylene coordination to the metal centre but does not encumber both axial coordination site. So the polymerization performance of 1 can be addressed to the catalytic pocket generated by the coordinated ligand that favors both chain transfer and chain walking over propagation.


 Please wait while we load your content...
Please wait while we load your content...