Ring-opening polymerization of donor–acceptor cyclopropanes catalyzed by Lewis acids†
Abstract
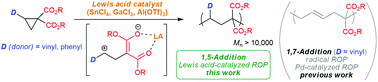
Ring-opening polymerization (ROP) of cyclopropane derivatives catalyzed by Lewis acids has been realized for the first time. In contrast to the conventional radical ROP of dialkyl 2-vinylcyclopropane-1,1-dicarboxylates that proceeds via 1,7-addition, the ROP catalyzed by SnCl4 under ambient conditions selectively affords 1,5-addition polymers with Mn up to 12 600 in good to high yields. The high selectivity of the 1,5-addition structure can be explained by the formation of six-membered transition states from propagating enols and monomers. The obtained polymers show higher glass transition temperatures and better solubilities than the 1,7-addition polymer. The ROP of dimethyl 2-phenylcyclopropane-1,1-dicarboxylate also occurred with SnCl4 as the catalyst in CH3NO2. Donor (vinyl or phenyl) and acceptor (two esters) substituents at the 1,2-positions of cyclopropanes are required to promote an efficient ROP, as the Lewis acids coordinate the two ester groups of the monomers to generate a 1,3-dipole intermediate stabilized by their substituents. This Lewis acid catalysis allows access to polymers that cannot be synthesized by previous radical, anionic, and Pd-catalyzed ROPs.


 Please wait while we load your content...
Please wait while we load your content...