Acceleration and improved control of aqueous RAFT/MADIX polymerization of vinylphosphonic acid in the presence of alkali hydroxides†
Abstract
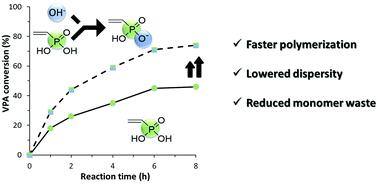
The effect of adding various alkali hydroxides to the conventional and reversible RAFT/MADIX radical polymerizations of vinylphosphonic acid (VPA) has been investigated. The addition of up to 1 equivalent of NaOH increases the rate and the final conversion of both conventional and RAFT/MADIX polymerizations. Larger quantities of NaOH retard the polymerization. LiOH, KOH and NH4OH also increase the rate of polymerization, to a lesser extent. In all cases, the most pronounced effect is observed in the presence of 0.5 equivalents of hydroxide relative to VPA. In RAFT/MADIX polymerizations, the dispersity of the final polymer decreases as the ionic radius of the counterion increases (H+ > Li+ > Na+ > K+ > NH4+), while the acceleration of polymerization follows the order Na+ > K+ > NH4+ > Li+ > H+. Thus the use of 0.5 eq. NaOH leads to the fastest polymerizations, while 0.5 eq. KOH and NH4OH provide a moderate increase in rate coupled with a significant reduction in dispersity of the final polymer.


 Please wait while we load your content...
Please wait while we load your content...