A thioglycerol route to bio-based bis-cyclic carbonates: poly(hydroxyurethane) preparation and post-functionalization†
Abstract
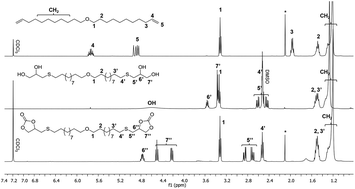
The present work is dedicated to the design of novel sulfur-substituted cyclic carbonates from thioglycerol, fatty acids and sugar derivatives. In this methodology, a sulfur atom is inserted in the β position of 5-membered ring cyclic carbonates via a two-step synthesis including the thiol–ene coupling of thioglycerol on fatty acid derivatives, followed by a transcarbonation. A similar strategy was adopted to prepare glycolipid-based cyclic carbonates in order to bring biodegradability to the final poly(hydroxyurethane)s. The so-formed monomers were characterized by NMR spectroscopies, HPLC and DSC. The enhanced reactivity of sulfur-substituted cyclic carbonates was demonstrated through a 1H NMR spectroscopy kinetic study of a model reaction with hexylamine. Fatty acid- and glycolipid-based sulfur-substituted bis-cyclic carbonates were then polymerized with diamines in a solvent using a catalyst-free process. FTIR, NMR, SEC, DSC and TGA were performed to investigate the PHUs’ chemical structure, molar masses and thermal properties. Finally, the so-formed PHUs were post-functionalized by sulfonation with m-CPBA taking advantage of thioether functions. The impact of the chemical modification was mostly studied on the polymer solubility and thermal stability.


 Please wait while we load your content...
Please wait while we load your content...