Facile green synthesis of isoindigo-based conjugated polymers using aldol polycondensation†
Abstract
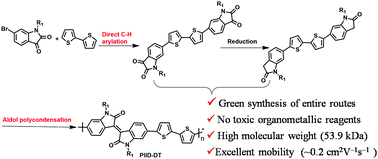
The aldol reaction is a facile green synthetic method and has been widely used in the synthesis of small molecules. In this study, we attempted to prepare conjugated polymers using aldol polymerization for the first time. Two isoindigo-based conjugated polymers (PIID-DT and PBIBDF-DT) were synthesized successfully in excellent yields and with high molecular weights (MGPCn up to 53.9 kDa) using toluene as the solvent and an acid as the catalyst. The entire synthesis route is economical and environmentally friendly, and the traditional drawbacks such as the use of organometallic reagents, toxic tin monomers, and other environmentally harmful compounds often encountered in Stille and Suzuki cross-coupling reactions could be avoided. Moreover, the isoindigo-based polymers prepared by the newly established aldol polymerization were also evaluated as organic field-effect transistors with bottom-gate/top-contact devices and exhibited excellent mobilities as high as 0.16 and 0.26 cm2 V−1 s−1 with a high Ion/Ioff ratio of 105 for PIID-DT and PBIBDF-DT, respectively. Therefore, aldol polycondensation has environmentally friendly characteristics that can be applied in the green synthesis of isoindigo-based conjugated polymers.


 Please wait while we load your content...
Please wait while we load your content...