Synthesis of highly reactive polyisobutylene by catalytic chain transfer in hexanes at elevated temperatures; determination of the kinetic parameters†
Abstract
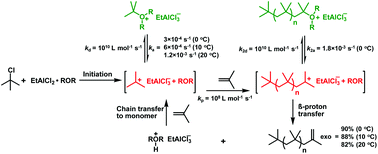
The kinetics and mechanism of the polymerization of isobutylene (IB) using an ethylaluminum dichloride (EADC)·bis(2-chloroethyl) ether (CEE) complex as a catalyst in conjunction with tert-butyl chloride (t-BuCl) as an initiator in hexanes at 0 °C have been previously reported. In an effort to further study the catalyst performance, we have investigated the polymerization at elevated temperatures. Polymerization rates increased while molecular weights and exo-olefin contents (90–78%) decreased with increasing temperature. At elevated temperatures the first-order plots are curved upward, suggesting that the formation of the tert-butyloxonium ion is slower at higher temperatures. 1H NMR studies confirmed that the t-butyloxonium ion is stable up to 15 °C but slowly decomposes at 20 °C. Linear first order plots were obtained when the polymerization was carried out with the tert-butyloxonium ion preformed at 10 °C. The slope of the first order plots that is proportional to the steady state concentration of carbenium ions increased 2, 3 and 4 fold at 10, 15 and 20 °C relative to that at 0 °C. Kinetic parameters of activation–deactivation were determined using model reactions. The rate constant of activation at 0 °C (ka = 3 × 10−4 s−1) increased 2, 3.4 and 4 fold at 10, 15 and 20 °C, respectively, in line with the rate increases. The deactivation rate constant, kd = 1010 L mol−1 s−1 was at the diffusion-limit.


 Please wait while we load your content...
Please wait while we load your content...