Direct arylation polymerization of asymmetric push–pull aryl halides†
Abstract
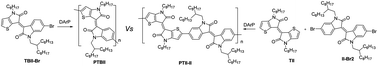
Recently, direct arylation polymerization (DArP) has emerged as a greener polymerization method for donor–acceptor (D–A) type conjugated polymers. DArP, in contrast to Stille or Suzuki polymerization, does not involve organometallic compounds as nucleophiles. This feature makes it feasible to directly use asymmetric aryl halides as monomers for preparing D–A polymers. In this study, we report the design of an asymmetric push–pull thieno-benzo-isoindigo (TBII) based monomer and explore the potential of DArP of TBII. With careful studies on catalytic systems and reaction conditions, we successfully prepared TBII based polymers with high molecular weights. As a control experiment, we also attempted to prepare the symmetric polymer thienoisoindigo-co-isoindigo (PTII-II) via DArP. The result suggested that the use of H-DA-X type monomers is advantageous to achieve high molecular weights in DArP.


 Please wait while we load your content...
Please wait while we load your content...