Tandem modification of amphiphilic cellulose ethers for amorphous solid dispersion via olefin cross-metathesis and thiol-Michael addition†
Abstract
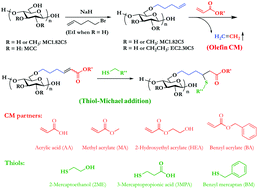
Olefin cross-metathesis (CM) has been shown to be a valuable, versatile strategy for modifications of cellulose derivatives with appended olefin “handles”. This synthetic method provides access to polysaccharide derivatives with a diverse assortment of functional groups under very mild conditions and with high efficiency. One potentially problematic aspect of the initial α,β-unsaturated CM products is their tendency to undergo free radical abstraction of hydrogen atoms γ to the introduced unsaturated carbonyl, which may lead to polymer crosslinking and loss of solubility. In order to eliminate this instability, we showed previously that diimide hydrogenation reliably removes the α,β-unsaturation, affording carboxyl-containing amphiphilic cellulose ethers that are promising candidates for amorphous solid dispersion (ASD). In this work, we show how to exploit, rather than eliminate the reactivity of the α,β-unsaturated CM products, employing thiol-Michael addition. Addition of a thiol to the conjugated olefin not only eliminates the cross-linking tendency, but also incorporates new functionality, thereby providing a doubly functional, branched polymer side chain. Due to the fact that we chose to append these substituents by hydrolytically stable ether tethers, we could also saponify selected ester-terminal CM and thiol-Michael addition products to provide an additional carboxyl group. Preliminary drug crystallization experiments were also performed with the model drug telaprevir, and these newly synthesized amphiphilic, branched, carboxypentyl cellulose ethers were shown to be efficient inhibitors of drug crystallization. This facile, efficient overall method enables synthesis of a collection of amphiphilic, branched cellulose ether derivatives with multiple functional groups, feeding detailed structure–property relationship studies for applications including ASD.


 Please wait while we load your content...
Please wait while we load your content...