Synthesis and side-chain isomeric effect of 4,9-/5,10-dialkylated-β-angular-shaped naphthodithiophenes-based donor–acceptor copolymers for polymer solar cells and field-effect transistors†
Abstract
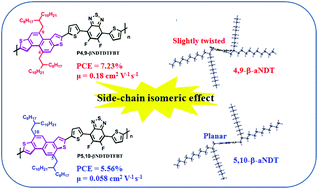
A systematic methodology is developed to construct the angular-shaped β-form naphthodithiophene (β-aNDT) core with regiospecific substitution of two alkyl groups at its 4,9- or 5,10-positions via the base-induced double 6π-cyclization of dithienyldieneyne precursors, leading to the two isomeric 4,9-β-aNDT and 5,10-β-aNDT monomers. It is found that a more curved geometry of the β-aNDT units intrinsically increases the solubility and thus the solution-processability of the resultant polymers. Therefore, β-aNDT units are ideal for polymerization with an acceptor-containing monomer without the need for any solubilizing aliphatic side chains, which are considered the insulating portion that jeopardizes charge transport. Based on this consideration, the 4,9- and 5,10-dialkylated β-aNDT monomers are polymerized with the non-alkylated DTFBT acceptor to afford two P4,9-βNDTDTFBT and P5,10-βNDTDTFBT copolymers for head-to-head comparison of the 4,9-inner/5,10-outer isomeric alkylation effect. It is found that 4,9-β-aNDT adopts a twisted conjugated structure due to the intramolecular steric repulsion between the inner branched side chains and the β-hydrogens on the thiophene rings. The slightly twisted 4,9-β-aNDT moiety allows P4,9-βNDTDTFBT to have higher solubility upon polymerization and thus a higher molecular weight, which eventually induces a higher ordered packing structure in the thin film compared to P5,10-βNDTDTFBT. As a result, P4,9-βNDTDTFBT exhibits a higher OFET mobility of 0.18 cm2 V−1 s−1, and the P4,9-βNDTDTFBT:PC71BM-based solar cell device also achieves a higher PCE of 7.23%, which is even better than the corresponding P4,9-αNDTDTFBT-based device.


 Please wait while we load your content...
Please wait while we load your content...