The impact of the molecular weight on the electrochemical properties of poly(TEMPO methacrylate)†
Abstract
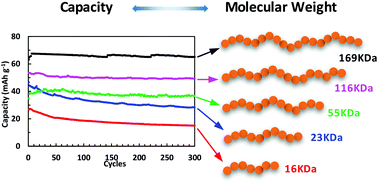
Synthesis of TEMPO-containing polymers by ‘living’ radical polymerization provides opportunities to investigate the impact of the molecular weight on their electrochemical properties. In this work, we utilized single electron transfer-living radical polymerization (SET-LRP) to synthesize poly(2,2,6,6-tetramethylpiperidine methacrylate) (PTMPM) with degrees of polymerization ranging from 66 to 703, and after oxidation producing poly(TEMPO methacrylate) (PTMA) with the highest molecular weight of 169 kDa and dispersity of 1.35. Three different techniques were used to quantify the radical density and calculate the theoretical capacity of PTMA polymers. These PTMA polymers, as active materials with 25 wt% in the electrode composite, showed strong molecular weight dependence on the electrochemical properties. The higher molecular weight PTMA polymers showed higher specific discharging capacities and better cycling stability due to their lower solubility in the electrolyte.


 Please wait while we load your content...
Please wait while we load your content...