Polymerization of α-(halomethyl)acrylates through sequential nucleophilic attack of dithiols using a combination of addition–elimination and click reactions†
Abstract
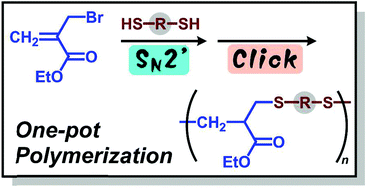
Polymerization of α-(halomethyl)acrylates and dithiols was achieved by the combination of SN2′ (addition–elimination) and subsequent thiol–ene click reactions. A Bu3P catalyst and an excess amount of a base that does not yield an acidic salt, e.g., 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or K2CO3, were key for achieving Mn > 104.


 Please wait while we load your content...
Please wait while we load your content...