CO2-Responsive graft copolymers: synthesis and characterization†
Abstract
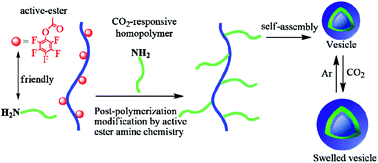
A facile and versatile approach for preparing CO2-responsive graft copolymers via a grafting-to strategy that combines controlled radical polymerization with post-polymerization modification has been demonstrated. A well-defined poly(N,N-dimethylacrylamide-co-pentafluorophenyl acrylate) (P(DMA-co-PFPA)) was synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization bearing reactive pentafluorophenyl esters as grafting sites on a hydrophilic backbone. Then, amino end-functionalized poly(2-(diethylamino)ethyl methacrylate) (PDEAEMA) known for its CO2 responsiveness and hydrophobic poly(methyl methacrylate) (PMMA) were prepared via atom transfer radical polymerization (ATRP) as side blocks, respectively. Subsequently, post-polymerization modification via active-ester amine chemistry, resulted in CO2-responsive graft copolymers with either homo-side chains or discrete multi-functional hetero side chains. Importantly, this grafting-to strategy utilizing active-ester amine chemistry appeared to be a facile and efficient method to fabricate graft copolymers. Interestingly, both prepared CO2-responsive graft copolymers – due to their amphiphilic nature – could self-assemble into vesicles in aqueous solution and displayed CO2-responsive expansion owing to the protonation of tertiary amine groups of PDEAEMA side chains under CO2 stimulation.


 Please wait while we load your content...
Please wait while we load your content...