1,4-Specific copolymerization of 1,3-cyclohexadiene with isoprene and their terpolymerization with styrene by cationic half-sandwich fluorenyl rare-earth metal alkyl catalysts†
Abstract
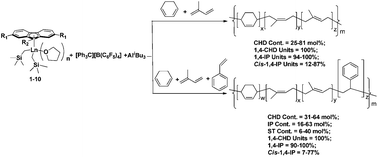
1,4-Specific copolymerization of 1,3-cyclohexadiene (CHD) with isoprene (IP) and their terpolymerization with styrene (S) have been achieved for the first time by cationic half-sandwich fluorenyl rare-earth metal alkyl catalysts in situ generated from half-sandwich fluorenyl rare-earth metal dialkyl complexes Flu′Ln(CH2SiMe3)2(THF)n (1–10) in combination with an activator ([Ph3C][B(C6F5)4] (A), [PhNHMe2][B(C6F5)4] (B) and B(C6F5)3 (C)) and AliBu3. The copolymerization of CHD with IP affords 1,4-selective random CHD–IP copolymers containing different comonomer contents and sequence distributions unavailable previously (CHD content = 25–81 mol%, 1,4-CHD unit = 100%; 1,4-IP unit = 94–100%; cis-1,4-IP unit = 12–87%). Moreover, new 1,4-specific random CHD–IP–S terpolymers with different compositions and sequence distributions (CHD content = 31–64 mol%, IP content = 16–63 mol%, S content = 6–40 mol%, 1,4-CHD unit = 100%; 1,4-IP unit = 90–100%, cis-1,4-IP unit = 7–77%) are obtained via the terpolymerization of CHD, IP, with S. The activity and regio-/stereoselectivity of copolymerization as well as the comonomer content, sequence distribution, molecular weight and molecular weight distribution of copolymer can be easily controlled by modifying the substituted fluorenyl ligand, metal center, activator and molar ratio of comonomers. Residual C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the random CHD–IP copolymers and CHD–IP–S terpolymers are completely epoxidized by meta-chloroperoxybenzoic acid (mCPBA) at room temperature, affording high-performance copolymers with polar groups and reactive sites in the polymer backbone.
C bonds of the random CHD–IP copolymers and CHD–IP–S terpolymers are completely epoxidized by meta-chloroperoxybenzoic acid (mCPBA) at room temperature, affording high-performance copolymers with polar groups and reactive sites in the polymer backbone.


 Please wait while we load your content...
Please wait while we load your content...