A facile synthetic route to stereoregular helical poly(phenyl isocyanide)s with defined pendants and controlled helicity†
Abstract
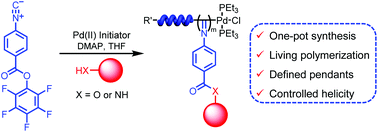
The living polymerization of a pentafluorophenyl (PFP) ester-functionalized phenyl isocyanide (1) using an alkyne–Pd(II) initiator affords well-defined poly(phenyl isocyanide)s with controlled molecular weights (Mns) and narrow molecular weight distributions (Mw/Mns). Post-polymerization modification of these polymers using alcohols and amines formed poly(phenyl isocyanide)s with defined ester and amide pendants. However, the polymers are optically inactive even when chiral alcohols and amines were used in the post-modification reactions. Interestingly, living polymerization of 1 using the Pd(II) initiator can be carried out in the presence of alcohols and amines in one-pot. Such one-pot polymerizations lead to the formation of well-defined helical poly(phenyl isocyanide)s in high yields with defined pendants through the linkage of an ester or amide. Remarkably, optically active helical poly(phenyl isocyanide)s with preferred handedness were facilely obtained when chiral alcohols and amines were used in one-pot polymerizations. The optical activities of the afforded helical polyisocyanides were relatively high and comparable to those prepared via polymerization of the corresponding enantiopure phenyl isocyanide monomers bearing chiral ester or amide groups.

 Please wait while we load your content...
Please wait while we load your content...