Donor–acceptor conjugated polymers based on two-dimensional thiophene derivatives for bulk heterojunction solar cells†
Abstract
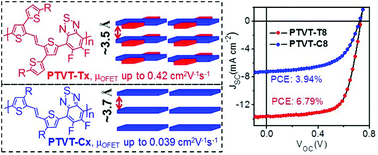
Four donor–acceptor (D–A) conjugated polymers (CPs), i.e., poly[(E)-1,2-bis(5-(2-hexyldecyl)-[2,3′-bithiophen]-2′-yl)ethene-alt-5,6-difluorobenzo[c][1,2,5]thiadiazole] (PTVT-T8), poly[(E)-1,2-bis(5-(2-octyldodecyl)-[2,3′-bithiophen]-2′-yl)ethene-alt-5,6-difluorobenzo[c][1,2,5]thiadiazole] (PTVT-T10), poly[(E)-1,2-bis(3-(2-hexyldecyl)thiophen-2-yl)ethene-alt-5,6-difluorobenzo[c][1,2,5]thiadiazole] (PTVT-C8) and poly[(E)-1,2-bis(3-(2-octyldodecyl)thiophen-2-yl)ethene-alt-5,6-difluorobenzo[c][1,2,5]thiadiazole] (PTVT-C10) were synthesized and characterized. Compared to PTVT-C8 and PTVT-C10, PTVT-T8 and PTVT-T10 that comprise two-dimensional (2D) conjugated D units displayed lower bandgaps, more rigid backbones, higher self-organization ability and shorter π–π stacking distances (∼3.5 Å). As a result, the hole mobility of PTVT-T8 and PTVT-T10 (up to 0.42 cm2 (V s)−1) was one magnitude higher than that of PTVT-C8 and PTVT-C10 (up to 0.039 cm2 (V s)−1), as measured with organic field-effect transistors (OFETs). Polymer solar cells (PSCs) of these polymers were prepared with PC71BM as an acceptor and o-xylene as a processing solvent, and power conversion efficiencies (PCEs) up to 6.79%, 5.65%, 3.94% and 1.70% were achieved for PTVT-T8, PTVT-T10, PTVT-C8 and PTVT-C10, respectively. PTVT-T8 and PTVT-T10 exhibited photovoltaic properties superior to PTVT-C8 and PTVT-C10 owing to their high hole-mobility and the formation of ordered nano-fibrillar structures in which polymer backbones preferred face-on arrangement.

 Please wait while we load your content...
Please wait while we load your content...