New insight into the polymerization mechanism of 1,3-dienes cationic polymerization. IV. Mechanism of unsaturation loss in the polymerization of isoprene†
Abstract
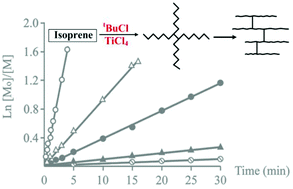
The cationic polymerization of isoprene with the tBuCl/TiCl4 initiating system in the presence of a high excess of tBuCl over TiCl4 ([tBuCl]/[TiCl4] > 100) in CH2Cl2 is reported. It is shown that the polymerization follows first-order kinetics, which indicates that the main chain-breaking process is the chain transfer to the initiator. The number-average functionalities with respect to the tert-butyl head group and chlorine-containing end group are determined to be considerably higher than unity. In addition, unsaturation of the polymer chain is found to be always less than 100%. It is also shown that unsaturation of the polyisoprene chain decreases with a simultaneous increase in polydispersity upon treatment of the polymer using the tBuCl/TiCl4 initiating system. Based on these observations, the mechanism to obtain a polymer with reduced unsaturation is proposed, which includes multiple interactions of growing cations with double bonds of “own” or/and “alien” macromolecules with the formation of branched structures.

 Please wait while we load your content...
Please wait while we load your content...