A new Schiff-base chemosensor for selective detection of Cu2+ and Co2+ and its copper complex for colorimetric sensing of S2− in aqueous solution†
Abstract
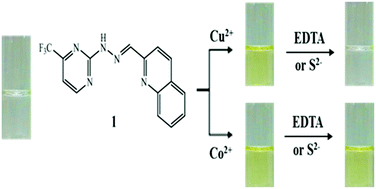
A new Schiff-base colorimetric chemosensor 1 was developed for the detection of Cu2+, Co2+ and S2−. Sensor 1 could simply monitor Cu2+ and Co2+ by a color change from colorless to yellow. The binding modes of 1 to Cu2+ and Co2+ were determined to be a 2 : 1 complexation stoichiometry through Job's plot and ESI-mass spectrometry analysis. The detection limits (0.02 μM and 0.63 μM) for Cu2+ and Co2+ were lower than the recommended values (31.5 μM and 1.7 μM) by the World Health Organization (WHO) for Cu2+ and the Environmental Protection Agency (EPA) for Co2+, respectively. Importantly, 1 could detect and quantify Cu2+ in real water samples. In addition, the Cu2+-2·1 complex could be used as a highly selective colorimetric sensor for S2− in the presence of other anions without any interference. Moreover, the sensing mechanisms of Cu2+ and Co2+ by 1 were explained by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...