Aerobic oxidative acylation of nitroarenes with arylacetic esters under mild conditions: facile access to diarylketones†
Abstract
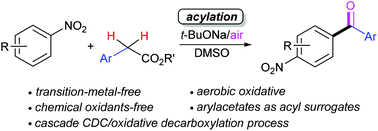
A facile and regioselective base-mediated aerobic oxidative acylation of nitroarenes to access diarylketones under mild conditions has been developed. It features the use of bench-stable and readily available arylacetates as acyl surrogates, and the absence of transition-metals and synthetic oxidants. This protocol involves a cascade CDC/oxidative decarboxylation process.


 Please wait while we load your content...
Please wait while we load your content...