Photochemical and oxidative cyclisation of tetraphenylpyrroles†
Abstract
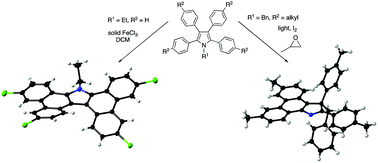
The photochemical and oxidative cyclodehydrogenation reactions of tetraphenylpyrroles act in a complementary fashion for the cyclisation of N-ethyl and N-benzyl derivatives. In the case of the former, a doubly cyclised product was isolated from cyclisation with solid FeCl3, while the latter gives a rearranged 3H-pyrrole upon irradiation.


 Please wait while we load your content...
Please wait while we load your content...