Exploration of a Au(i)-mediated three-component reaction for the synthesis of DNA-tagged highly substituted spiroheterocycles†
Abstract
We demonstrate a Au(I)-mediated three-component reaction to DNA-tagged highly substituted 6-oxa-1,2-diazaspiro[4.4]nonanes from either DNA-coupled aldehydes, hydrazides, or alkynols. The choice of the starting material coupled to the DNA tag was critial for the purity of the product as the DNA-aldehyde conjugate yielded the purest products, whereas the alkynol- and hydrazide conjugates returned complex product mixtures. The reaction was compatible with thymine-, cytosine-, and, surprisingly, with adenine-DNA, while guanine-containing DNA strands were degraded under the reaction conditions.
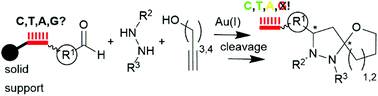
- This article is part of the themed collection: Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...