Extreme halophilic alcohol dehydrogenase mediated highly efficient syntheses of enantiopure aromatic alcohols†
Abstract
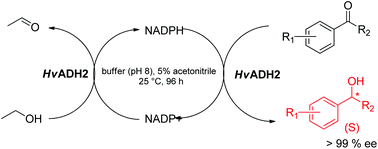
Enzymatic synthesis of enantiopure aromatic secondary alcohols (including substituted, hetero-aromatic and bicyclic structures) was carried out using halophilic alcohol dehydrogenase ADH2 from Haloferax volcanii (HvADH2). This enzyme showed an unprecedented substrate scope and absolute enatioselectivity. The cofactor NADPH was used catalytically and regenerated in situ by the biocatalyst, in the presence of 5% ethanol. The efficiency of HvADH2 for the conversion of aromatic ketones was markedly influenced by the steric and electronic factors as well as the solubility of ketones in the reaction medium. Furthermore, carbonyl stretching band frequencies ν (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) have been measured for different ketones to understand the effect of electron withdrawing or donating properties of the ketone substituents on the reaction rate catalyzed by HvADH2. Good correlation was observed between ν (C
O) have been measured for different ketones to understand the effect of electron withdrawing or donating properties of the ketone substituents on the reaction rate catalyzed by HvADH2. Good correlation was observed between ν (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of methyl aryl-ketones and the reaction rate catalyzed by HvADH2. The enzyme catalyzed the reductions of ketone substrates on the preparative scale, demonstrating that HvADH2 would be a valuable biocatalyst for the preparation of chiral aromatic alcohols of pharmaceutical interest.
O) of methyl aryl-ketones and the reaction rate catalyzed by HvADH2. The enzyme catalyzed the reductions of ketone substrates on the preparative scale, demonstrating that HvADH2 would be a valuable biocatalyst for the preparation of chiral aromatic alcohols of pharmaceutical interest.


 Please wait while we load your content...
Please wait while we load your content...