Synthetically useful variants of industrial lipases from Burkholderia cepacia and Pseudomonas fluorescens†
Abstract
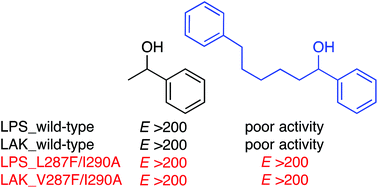
Industrial enzymes lipase PS (LPS) and lipase AK (LAK), which originate from Burkholderia cepacia and Pseudomonas fluorescens, respectively, are synthetically useful biocatalysts. To strengthen their catalytic performances, we introduced two mutations into hot spots of the active sites (residues 287 and 290). The LPS_L287F/I290A double mutant showed high catalytic activity and enantioselectivity for poor substrates for which the wild-type enzyme showed very low activity. The LAK_V287F/I290A double mutant was also an excellent biocatalyst with expanded substrate scope, which was comparable to the LPS_L287F/I290A double mutant. Thermodynamic parameters were determined to address the origin of the high enantioselectivity of the double mutant. The ΔΔH‡ term, but not the ΔΔS‡ term, was predominant, which suggests that the enantioselectivity is driven by a differential energy associated with intermolecular interactions around Phe287 and Ala290. A remarkable solvent effect was observed, giving a bell-shaped profile between the E values and the log P or ε values of solvents with the highest E value in i-Pr2O. This suggests that an organic solvent with appropriate hydrophobicity and polarity provides the double mutant with some flexibility that is essential for excellent catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...