Forsythenethosides A and B: two new phenylethanoid glycosides with a 15-membered ring from Forsythia suspensa†
Abstract
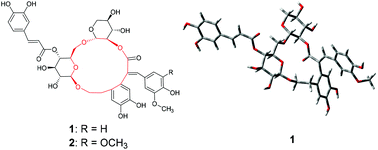
Forsythenethosides A (1) and B (2), two new phenylethanoid glycosides with an unprecedented 15-membered carbon scaffold ring, were isolated from the fruits of Forsythia suspensa. Their structures, including their geometric configurations, were determined via extensive NMR spectroscopy techniques, especially using 2D NMR data combined with systematic conformational analysis. Forsythenethosides A and B showed strong neuroprotective activities against serum-deprivation-induced PC12 cell damage. Furthermore, they were active on rotenone-induced PC12 cell damage.


 Please wait while we load your content...
Please wait while we load your content...