A hybrid molecule of a GFP chromophore analogue and cholestene as a viscosity-dependent and cholesterol-responsive fluorescent sensor†
Abstract
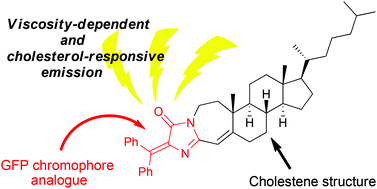
Diarylmethylenated and cholestene (or -tane)-hybrid analogues of the GFP chromophore, namely, Ch-DAINs were successfully synthesised by a condensation reaction between methyl imidates and N-(diarylmethylene)glycinates. Among the Ch-DAINs synthesised, a diphenyl-type analogue showed viscosity-dependent and cholesterol-responsive fluorescent properties. It showed a nearly linear increase of the fluorescence emission in triglycerides and vesicles as the amount of cholesterol was increased.


 Please wait while we load your content...
Please wait while we load your content...