The preference for dual-gold(i) catalysis in the hydro(alkoxylation vs. phenoxylation) of alkynes†
Abstract
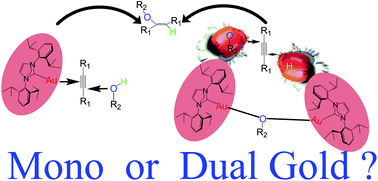
Dinuclear gold complexes and their use in catalysis have received significant recent attention, but there are few critical comparisons of mono- versus dual gold-catalysed pathways. Herein we study the hydroalkoxylation and hydrophenoxylation of alkynes using density functional theory calculations, and compare two possible mechanisms that have been proposed previously on the basis of theoretical and experimental studies, which unravel different preferences because of both the nature of the alkyne and alcohol and the non-innocent role of the counter-anion of a dual gold based catalyst. Entropy is found to have a significant effect, rendering the nucleophilic attack of the monoaurated intermediate [Au(L)(η2-alkyne)]+ difficult both kinetically and thermodynamically; this mechanism cannot easily form only the trans-alkene product that is observed experimentally. Instead, a reaction via a dual gold catalysed mechanism presents much lower barriers. In addition, for the sake of direct comparison with recent results by Belanzoni and Zuccaccia, oversimplification of the N-heterocyclic carbene (NHC) ligand in the calculations might decrease the enthalpy barrier and lead to results that are not directly applicable to experiments. Moreover, the alkylic or arylic nature of the alkyne and/or alcohol is also tested.


 Please wait while we load your content...
Please wait while we load your content...