α-Halo carbonyls enable meta selective primary, secondary and tertiary C–H alkylations by ruthenium catalysis†
Abstract
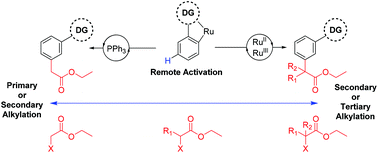
A catalytic meta selective C–H alkylation of arenes is described using a wide range of α-halo carbonyls as coupling partners. Previously unreported primary alkylations with high meta selectivity have been enabled by this methodology whereas using straight chain alkyl halides affords ortho substituted products. Mechanistic analysis reveals an activation pathway whereby cyclometalation with a ruthenium(II) complex activates the substrate molecule and is responsible for the meta selectivity observed. A distinct second activation of the coupling partner allows site selective reaction between both components.


 Please wait while we load your content...
Please wait while we load your content...