Direct access to isoxazolino and isoxazolo benzazepines from 2-((hydroxyimino)methyl)benzoic acid via a post-Ugi heteroannulation†
Abstract
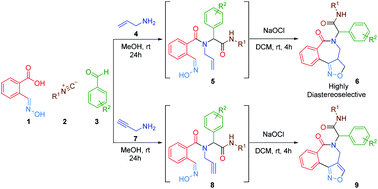
A diversity-oriented access to isoxazolino and isoxazolo benzazepines is elaborated via a post-Ugi heteroannulation involving intramolecular 1,3-dipolar cycloaddition reaction of nitrile oxides with alkenes and alkynes. This sequence offers an interesting multicomponent entry to a library of isoxazolino and isoxazolo benzazepines under mild reaction conditions in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...