The synthesis of polysubstituted indoles from 3-bromo-2-indolyl phosphates†
Abstract
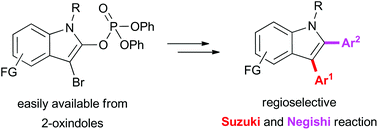
A novel methodology for the synthesis of functionalised indoles based on the cross-coupling reactions of 3-bromo-2-indolyl phosphates is described. The preparation involves the conversion of easily available 2-oxindoles to 3,3-dibromo-2-oxindoles followed by the Perkow reaction affording 3-bromo-2-indolyl phosphates. Then bromine atom is substituted regioselectively by the Suzuki coupling reaction. We observed that aluminum chloride promoted the reaction of 3-substituted-2-indolyl phosphates with organozinc reagents furnishing 2,3-disubstituted indoles as final products. The overall diversity and efficiency of the methodology was demonstrated by the synthesis of bioactive molecule from easily available substances.


 Please wait while we load your content...
Please wait while we load your content...