Endomorphin-1 analogs containing α-methyl-β-amino acids exhibit potent analgesic activity after peripheral administration†
Abstract
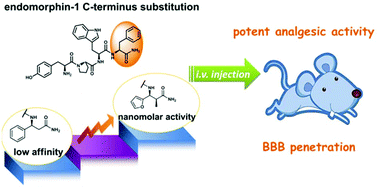
This study describes the design and synthesis of endomorphin-1 analogs containing C-terminal aromatic α-methyl-β-amino acids and an N-terminal native tyrosine or 2,6-dimethyl-tyrosine. We show that, in comparison with the parent peptide, these analogs exhibit improved bioactivity and blood–brain barrier penetration after intravenous administration, and have a lower tendency to induce constipation and sedation than morphine.


 Please wait while we load your content...
Please wait while we load your content...