DDQ-promoted direct C5-alkylation of oxazoles with alkylboronic acids via palladium-catalysed C–H bond activation†
Abstract
A novel and concise C5-alkylation of oxazoles using alkylboronic acids as alkyl donors via Pd(II)-catalysed C–H bond activation has been achieved in moderate to good yields with satisfactory functional group tolerance. Instead of commonly used BQ as a key promoter, DDQ was discovered to be a better additive that significantly promoted this alkylation. This efficient and advanced method represents the first C(sp2)–C(sp3) cross-coupling reaction at the C5-position of oxazoles, which is particularly attractive due to its potential applications in the late-stage functionalization of oxazole-containing bioactive molecules.
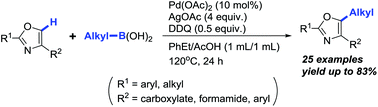


 Please wait while we load your content...
Please wait while we load your content...