SmI2-mediated reductive cyclization of β-arylthio ketones: a facile and diastereoselective synthesis of thiochroman derivatives†
Abstract
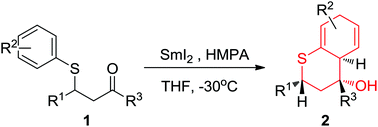
SmI2-mediated reductive cyclization of β-arylthio ketones to generate thiochroman derivatives is not a generally observed process and the reported examples are limited to geminal disubstitution in the substrates. The results of the current study show that the cyclization also occurs when other substitution patterns are present, affording a general approach to dihydrothiochroman-ols in good yields and high degrees of diastereoselectivity. Besides, the halogen substitution on β-aryl is tolerated in most cases here although reductive dehalogenation has been reported to predominate in the reductive cyclization process. Dihydrothiochroman-4-ols were readily oxidized to thiochroman-4-ols in almost quantitative yields.


 Please wait while we load your content...
Please wait while we load your content...