Transition metal mediated carbonylative benzannulations
Abstract
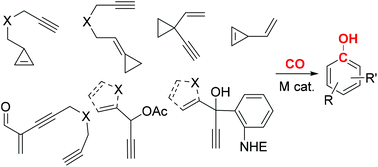
In carbonylative benzannulations, feedstock carbon monoxide is converted to a benzene ring, which is one of the most fundamentally important and common rings in natural products and pharmaceutical compounds. Carbon monoxide, however, is rather inert in the absence of transition metals. Historically, carbonylative benzannulations have been mediated by stoichiometric chromium and iron in the form of Fischer carbenes. Recently, a number of transition metal-catalyzed carbonylative benzannulations have been developed, and almost all of them involve rhodium catalysts. This review will briefly discuss the mechanism and applications of carbonylative benzannulations involving Fischer carbenes and compare them with the more recent transition metal-catalyzed processes, including [3 + 2 + 1] cycloadditions, [5 + 1] cycloadditions, and other less common cycloadditions.


 Please wait while we load your content...
Please wait while we load your content...