Ni and Cu-catalyzed one pot synthesis of unsymmetrical 1,3-di(hetero)aryl-1H-indazoles from hydrazine, o-chloro (hetero)benzophenones, and (hetero)aryl bromides†
Abstract
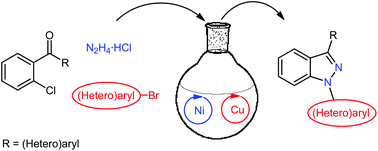
The nickel-catalyzed cyclization of in situ generated ortho-chlorobenzophenone hydrazone derivatives, to afford 3-(hetero)aryl-1H-indazoles, is documented for the first time. The product 1H-indazoles can be transformed subsequently in a one-pot procedure into 1,3-di(hetero)aryl-1H-indazoles via copper-catalyzed N-arylation with (hetero)aryl bromides.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...