A theoretical study of ascorbic acid oxidation and HOO˙/O2˙− radical scavenging†
Abstract
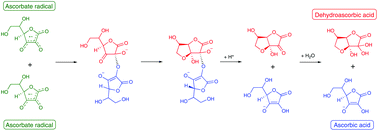
Ascorbic acid is a well-known antioxidant and radical scavenger. It can be oxidized by losing two protons and two electrons, but normally loses only one electron at a time. The reactivity of the ascorbate radical is unusual, in that it can either disproportionate or react with other radicals, but it reacts poorly with non-radical species. To explore the oxidation mechanism of ascorbic acid, the pKa's and reduction potentials have been calculated using the B3LYP/6-31+G(d,p) and CBS-QB3 levels of theory with the SMD implicit solvent model and explicit waters. Calculations show that the most stable form of dehydroascorbic acid in water is the bicyclic hydrated structure, in agreement with NMR studies. The possible oxidation reactions at different pH conditions can be understood by constructing a potential-pH (Pourbaix) diagram from the calculated pKa's and standard reduction potentials. At physiological pH disproportionation of the intermediate radical is thermodynamically favored. The calculations show that disproportionation proceeds via dimerization of ascorbate radical and internal electron transfer, as suggested by Bielski. In the dimer, one of the ascorbate units cyclizes. Then protonation and dissociation yields the fully reduced and bicyclic fully oxidized structures. Calculations show that this mechanism also explains the reaction of the ascorbic acid radical with other radical species such as superoxide. Ascorbate radical combines with the radical, and intramolecular electron transfer followed by cyclization and hydrolysis yields dehydroascorbic acid and converts the radical to its reduced form.


 Please wait while we load your content...
Please wait while we load your content...