A tunable copper-catalyzed multicomponent reaction towards alkaloid-inspired indole/lactam polycycles†
Abstract
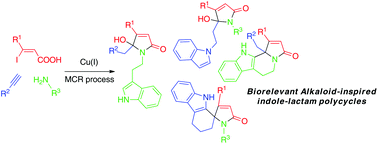
A versatile copper(I)-catalyzed cascade multicomponent reaction strategy between readily available (Z)-3-iodoacrylic acids, terminal alkynes, and primary amines is reported, leading to a great diversity of complex heterocyclic backbones based on biorelevant indole/lactam scaffolds.


 Please wait while we load your content...
Please wait while we load your content...