Synthesis of fused cyanopyrroles and spirocyclopropanes via addition of N-ylides to chalconimines†
Abstract
Addition of N-ylides derived from DABCO to chalconimines takes place through a Michael addition–cyclization pathway to afford fused cyanopyrroles and/or spirocyclopropanes. The product profile depends heavily on the nature of chalconimines. While 6-membered cyclic chalconimines provide a mixture of pyrrole and spirocyclopropane, 5-membered chalconimines furnish exclusively spirocyclopropane. Cyclopropane was the only product in the case of a representative open chain chalconimine as well. On the other hand, chalcones provide only spirocyclopropanes which is in contrast to the previously reported reactivity of enones. The complementary nature of the reactivity of the tetralone derived chalcone and its corresponding imine in providing spirocyclopropane and pyrrole, respectively, has also been demonstrated.
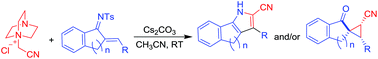


 Please wait while we load your content...
Please wait while we load your content...