Enantioselective total synthesis of (+)-arborescidine C and related tetracyclic indole alkaloids using organocatalysis†
Abstract
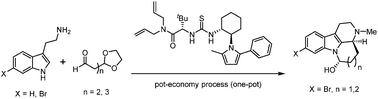
A concise and enantioselective synthesis of the tetracyclic indoles, including the naturally occurring compounds (+)-arborescidine C and (+)-arborescidine B, was achieved by the key step of Pictet–Spengler cyclization reaction with a Jacobsen-type thiourea organocatalyst. The synthetic process was further demonstrated in a pot-economy strategy and was achieved in a one-pot operation.


 Please wait while we load your content...
Please wait while we load your content...