Exploitation of new structurally diverse d-glucuronamide-containing N-glycosyl compounds: synthesis and anticancer potential†
Abstract
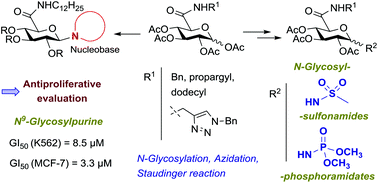
The synthesis and anticancer evaluation of novel N-glycosyl derivatives containing N-substituted glucuronamide moieties, as nucleoside analogs or as prospective mimetics of glycosyl phosphates or of nucleotides, is reported. These compounds comprise N-anomerically-linked nucleobases or motifs that are surrogates of a phosphate group, such as sulfonamide or phosphoramidate moieties. 1-Sulfonamido glucuronamides containing N-benzyl, N-propargyl or N-dodecyl carboxamide units were synthesized through glycosylation of methanesulfonamide with tetra-O-acetyl glucuronamides. 1-Azido glucuronamides were accessed by microwave-assisted reactions of tetra-O-acetyl glucuronamides with TMSN3 and were further converted into N-glycosylphosphoramidates by treatment with trimethyl phosphite. Potential glucuronamide-based nucleotide mimetics comprising both an anomeric sulfonamide/phosphoramidate group and a benzyltriazolylmethyl amide system at C-5, as nucleobase mimetics, were synthesized via ‘click’ cycloaddition of N-propargyl glucuronamide derivatives with benzyl azide. N-Dodecyl tetra-O-acetyl glucuronamides were converted into uracil and purine nucleosides via N-glycosylation of the corresponding silylated nucleobases. Biological screening revealed significant antiproliferative activities of the N-dodecyl glucuronamide-containing sulfonamide, phosphoramidate and nucleosides in K562 and MCF-7 cells. The highest effect was exhibited by the N9-linked purine nucleoside in the breast cancer cell MCF-7 with a GI50 value similar to that of clinically used 5-fluorouracil. Immunoblotting and cell cycle analysis of K562 cells treated with the most active compound as well as evaluation of the effect of this nucleoside on the activities of caspases 3 and 7 showed induction of apoptosis as the mechanism of cell death.


 Please wait while we load your content...
Please wait while we load your content...