New mechanistic interpretations for nitrone reactivity
Abstract
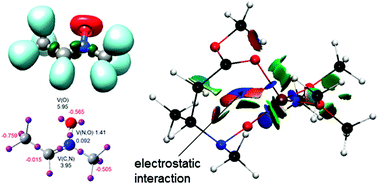
The reactivity of nitrones in cycloadditions and related reactions is revisited by introducing a topological perspective. In particular, the study of electron localization function (ELF) along a reaction pathway allows evaluating bond reorganization showing that in several cases the bonds are formed in a sequential way, the second one being formed once the first one is already formed. Both classical 1,3-dipolar cycloadditions and Mannich-type reactions revealed unexpected features often underestimated in classical mechanistic studies.


 Please wait while we load your content...
Please wait while we load your content...