In situ formation of pyronin dyes for fluorescence protease sensing†
Abstract
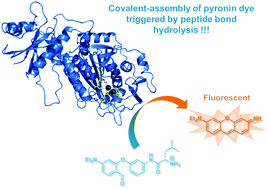
We report a reaction-based strategy for the fluorogenic detection of protease activity. Based on the “covalent-assembly” probe design principle recently put forward by the Yang group for detection of Sarin related threats (J. Am. Chem. Soc., 2014, 136, 6594–6597), we have designed two unusual non-fluorescent caged precursors (mixed bis-aryl ethers) which are readily converted into a fluorescent unsymmetrical pyronin dye through a domino cyclisation–aromatisation reaction triggered by penicillin G acylase (PGA) or leucine aminopeptidase (LAP). Fluorescence-based in vitro assays and HPLC-fluorescence/-MS analyses support the claimed activation mechanism whose the further implementation to “smart” imaging agents for the study of protease function in vivo is expected.


 Please wait while we load your content...
Please wait while we load your content...