Synthesis of 2-aryloxy butenoates by copper-catalysed allylic C–H carboxylation of allyl aryl ethers with carbon dioxide†
Abstract
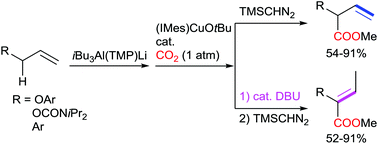
Efficient synthesis of 2-aryloxy-3-butenoic acid esters by allylic C–H bond carboxylation of allyl aryl ethers with CO2 has been achieved through deprotonative alumination with an aluminium ate compound (iBu3Al(TMP)Li) followed by NHC-copper-catalysed carboxylation of the resulting aryloxy allylaluminum species. Functional groups such as halogens (F, Cl, Br, I), CF3, amino, methylthio, silyloxy and hetero aromatic groups survived the reaction conditions. The regio- and stereoselective transformation (isomerization) of 2-aryloxy-3-butenoate products to (Z)-2-aryloxy-2-butenate isomers has also been achieved in the presence of a catalytic amount of DBU. These transformations thus constitute an efficient protocol for the divergent synthesis of both 2-aryloxy-3- and 2-butenonates from a single allyl aryl ether substrate using CO2 as a C1 building block.


 Please wait while we load your content...
Please wait while we load your content...