Enantioselective copper catalysed intramolecular C–H insertion reactions of α-diazo-β-keto sulfones, α-diazo-β-keto phosphine oxides and 2-diazo-1,3-diketones; the influence of the carbene substituent†
Abstract
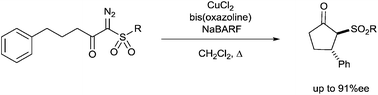
Enantioselectivities in C–H insertion reactions, employing the copper-bis(oxazoline)-NaBARF catalyst system, leading to cyclopentanones are highest with sulfonyl substituents on the carbene carbon, and furthermore, the impact is enhanced by increased steric demand on the sulfonyl substituent (up to 91%ee). Enantioselective intramolecular C–H insertion reactions of α-diazo-β-keto phosphine oxides and 2-diazo-1,3-diketones are reported for the first time.
- This article is part of the themed collection: Celebrating Excellence in Research: 100 Women of Chemistry


 Please wait while we load your content...
Please wait while we load your content...